ACUTRON

- ‹ vorige
- 21 van de 1068
- volgende ›
http://www.eastwestmed.com/guidedtour_1.php ![]()
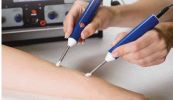
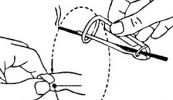
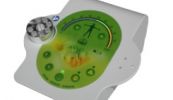
The Acutron Mentor is not just a microcurrent device. It's approved by the FDA and packaged with solutions including 19 built in treatment protocols, easy to use controls, microcurrent and milliamp capable, one-on-one training, and sales & marketing guidelines. It's a success system!
Bij de Acutron gaat het om een clearance en zeker niet over een approval.
Een clearance van de minste soort, want het apparaat kan niet worden verboden omdat het in principe identiek is aan een voorganger van voor 1976 en geen gevaar oplevert.
Zie de FDA-brief http://www.accessdata.fda.gov/cdrh_docs/pdf/K981976.pdf ![]()
die stelt en detailleert dat de Acutron merendeels gelijk is aan de tot voor 1976 teruggaande modellen ACUTRON MULTIWAVE, THERATOUCH en de DYNATRON
Duidelijker draait het om het aandikken van inkomens:
Consider these 5 questions:
(1) Do you need to augment your income through increased new patient referrals?
(2) Would you like to have a clinical tool that will "never be old" technology?
(3) Are you frustrated with hard-to-treat patient pain issues?
(4) When you have questions, don't you want immediate answers?
(5) Would you like a device that has flexibility and will grow with your knowledge and capabilities?
. … . .
If the answer to any of these questions is yes, please call the Eastwestmed sales department at 1-800-872-6789 to schedule a complimentary business evaluation and briefing session for the individual needs of your practice. Please continue on with the tour.
zie ook: https://skepsis.nl/museum/nl/algemene-info/fda.html
http://www.casewatch.org/fdawarning/prod/2001/tens.shtml ![]()
The Acutron Mentor was cleared under section 510(k) of the Act and is intended for the symptomatic relief of chronic intractable pain, post-traumatic pain, and post-surgical pain. Your website at www.microcurrentresearch.com/description.htm ![]() makes numerous claims for the Acutron Mentor that have not been cleared by the agency, i.e., healing acceleration of muscle and joint problems, muscle mass enhancement, meridian therapy, analgesic nerve block, reducing the need for surgery of the foot, knee, back, and TMJ, fibromyalgia, and Reflex Sympathetic Dystrophy (RSD).
makes numerous claims for the Acutron Mentor that have not been cleared by the agency, i.e., healing acceleration of muscle and joint problems, muscle mass enhancement, meridian therapy, analgesic nerve block, reducing the need for surgery of the foot, knee, back, and TMJ, fibromyalgia, and Reflex Sympathetic Dystrophy (RSD).
Additionally, your web site at www.microcurrentresearch.com/testimonials.htm ![]() contains many references and testimonial claims for the Acutron Mentor that have not been cleared by FDA.
contains many references and testimonial claims for the Acutron Mentor that have not been cleared by FDA.
Kortom let op hoe makkelijk de leverancier/behandelaar meer suggereert dan is toegestaan.