THERAMAG-usa

- ‹ vorige
- 964 van de 1068
- volgende ›
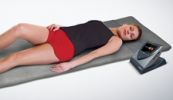
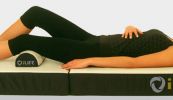
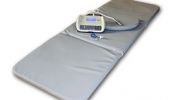
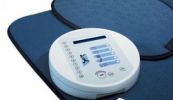
Bovenstaande THERAMAG-usa is duidelijk verschillend van het THERAMAG-magneet-matje.
09/2016 Website & Apparaat zijn niet terug te vinden.
http://www.theramag.com/product_description.htm ![]()
THERAMAG USA CORPORATION offers a unique device for helping people resolve various health related problems that might occur in everyday life. Our approach is based upon the principle of ‘systemic effects’ in interaction of the human body with electromagnetic fields.
In de bijlagen staat vermeld dat men bezig is goedkeuring van de FDA te verkrijgen.
Heden, april 2014, jan 2018, levert het intikken van "Theramag" in http://labels.fda.gov/company.cfm ![]() geen resultaten.
geen resultaten.
03/2019 Nog steeds niet.
Rustig afwachten wat het resultaat zal zijn, want dat deze magneettherapie meer zou betekenen dan welke andere ook, valt sterk te betwijfelen.
Kennelijk is er duidelijk een marketingplan voor gemaakt om de reguliere aanpak en de alternatieve in gescheiden verkoopkanalen te houden.
Het alternatieve deel kan worden verkocht als "Alleen voor onderzoek", waarmee het leveren van onderzoeksresultaten is omzeild.
http://www.bplans.com/medical_equipment_business_plan/executive_summary_fc.cfm ![]()
.
.
Strategy
MedNexis will target the following two markets: allopathic and alternative medicine. In order to effectively distance the two products from possible negative connotations associated with alternative medicine in the field of allopathic medicine, the two devices will be given separate and distinct names: MedStim for the allopathic medicine device and TheraMag for the alternative medicine device.
.
MedStim will be distributed through channels dominated by large distributors and strategic association with these players will be key to gaining acceptance in this market. Furthermore, physicians will demand randomized, controlled study data, the production of which will be the focus of the bulk of MedNexis' early efforts in this market.
.
TheraMag will be distributed to alternative medicine centers which are less centralized and direct sales will also be possible. Less scientific proof is required by this market, and entrance will be immediate once the FDA issues an Investigational Device Exemption.